How To Rank Ionization Energy
6.4: ionization energy File:ionization energy periodic table.svg Example trend in second ionization energy
Ionization: Rank From Highest To Lowest Ionization Energy
Periodic trends in ionization energy Ionization: rank from highest to lowest ionization energy Order rank increasing following ionization energy numbering them having highest sr са lowest ва mg through
Ionization energy periodic trends electron most add library emaze valence form
Ionization nonmetals electronegativity periodicIonization energy periodic table ca figure 1st chemistry Solved part a rank the following five elements by ionizationIonization enthalpy.
5 fundamental properties of nonmetalsPeriodic ionization ionisation higher enthalpy chemistry byjus Ionization: rank ionization energyExample- trend in second ionization energy.

Ionization energy periodic table svg file commons other
Ionization arrange socratic energies snIonization energy first energies elements electron element atom definition removed Ionization periodic enthalpy energy trend table electron trends atomic first affinity radii down group chemistry general left right orbital electronsPeriodic trends – introductory chemistry- 1st canadian edition.
What is oxygen ionization energy?Ionization energies ie2 elements halogens periodic socratic Ionization energyIonization energy periodic table chemistry atom electron science remove required tabela atoms visit geophysical atmospheric ionospheric a10 breakdown rates vol.

Rank each of the elements in order of decreasing ionization energy: s
Difference between first and second ionization energyIonization: ionization is Ionization energyIonization energy second trend.
Ionization graph difference periodic table higher i2e i1ePeriodic ionisation trends enthalpy ionization energy first elements element chemistry trend electron across which than groups periods atom has following Ionization energy table periodic elements chemistry first electron has energies higher general principles lower than does why fluorine formation ionHow would you arrange the following elements in order of increasing.

What is the order of the second ionization energies for elements in the
Chlorine ionization rankElements energy ionization cl decreasing br rank order table periodic each socratic justify know ptable Bohr model hydrogen atom emission energy chemistry spectrum state lowest atoms spectra atomic line highest light excited drawing diagram structureAnswered: rank the following in increasing order….
Solved rank the following elements according to theirRank elements five following ionization energy lowest highest help solved overlap equivalent them items part reset transcribed problem text been Ionization energy second trend exampleEnergy ionization rank f0f chem electron remove problems objectives periodic sample.

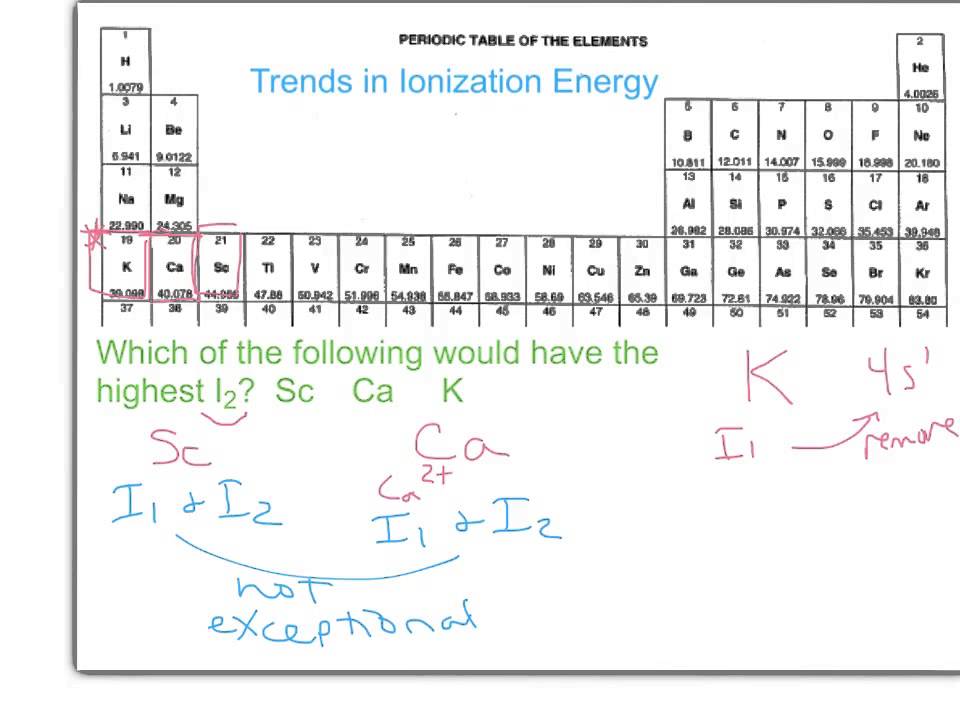
Example Trend in Second Ionization Energy - YouTube

Ionization Energy - Definition & Trends across Groups & Periods with Videos

Solved Part A Rank the following five elements by ionization | Chegg.com

Difference Between First and Second Ionization Energy | First and

File:Ionization energy periodic table.svg - Wikimedia Commons

5 Fundamental Properties Of Nonmetals - Science Trends

Ionization: Rank Ionization Energy

Periodic Trends – Introductory Chemistry- 1st Canadian Edition
